Top 30 Humira Manufacturers You Should Know
Are you struggling to find the best manufacturer for Humira? With so many options out there, it can feel overwhelming. Choosing the right supplier isn’t just about cost; it’s about quality, reliability, and trust. Imagine partnering with a factory that meets the highest standards, ensuring your product is safe and effective. The right choice can elevate your business and enhance patient satisfaction.
In this article, we’ve compiled a list of the top 30 Humira manufacturers, each with unique strengths and advantages. Ready to discover the best fit for your needs? Let’s dive in and find the perfect partner for your Humira production!
Top 30 Humira Manufacturer Manufacturers
Humira – Autoimmune Treatment Solutions
Domain: humira.com
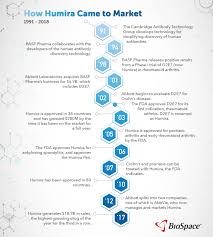
Registered: 2001 ( 24 years )
Introduction: Humira is a prescription medication used to treat various autoimmune conditions by inhibiting tumor necrosis factor (TNF).
Humirapro – Autoimmune Treatment Solutions
Domain: humirapro.com
Registered: 2006 ( 19 years )
Introduction: HUMIRA® (adalimumab) is a biologic medication used to treat various autoimmune conditions, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and more.
NPR – Healthcare Insights and Solutions
Domain: npr.org
Registered: 1993 ( 32 years )
Introduction: Humira is an injectable biologic treatment for rheumatoid arthritis and other autoimmune conditions. It has been a leading drug in its category for 20 years, generating approximately $200 billion in revenue.
Reliable Plant – Humira for Autoimmune Conditions
Domain: reliableplant.com
Registered: 2005 ( 20 years )
Introduction: HUMIRA (adalimumab) is a fully human monoclonal antibody approved for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and Crohn’s disease.
Pharmaceutical Technology – Autoimmune Disease Treatments
Domain: pharmaceutical-technology.com
Registered: 2000 ( 25 years )
Introduction: Humira is a monoclonal antibody drug developed by AbbVie, primarily used for treating autoimmune diseases.
Drugs.com – Biologic Medications for Autoimmune Diseases
Domain: drugs.com
Registered: 1998 ( 27 years )
Introduction: Humira is a biologic medication primarily used to treat various autoimmune diseases.
AbbVie Access – Autoimmune Treatment Support Solutions
Domain: abbvieaccess.com
Registered: 2021 ( 4 years )
Introduction: HUMIRA® (adalimumab) is a medication used to treat various autoimmune conditions. AbbVie provides support programs and resources for patients and healthcare professionals to facilitate access and adherence to treatment.
Nytimes – Health Insights and Treatment Information
Domain: nytimes.com
Registered: 1994 ( 31 years )
Introduction: Humira, a medication developed by AbbVie, primarily used for treating autoimmune diseases.
Ajmc – Autoimmune Disease Solutions
Domain: ajmc.com
Registered: 1997 ( 28 years )
Introduction: AbbVie’s adalimumab (Humira) is a top-selling pharmaceutical indicated for various autoimmune, rheumatologic, and gastrointestinal diseases.
AbbVie – Innovative Biopharmaceutical Solutions
GoodRx – Humira and Biosimilar Medication Solutions
Domain: goodrx.com
Registered: 2011 ( 14 years )
Introduction: Humira (adalimumab) is an injectable medication used to treat several autoimmune conditions. It has been available as a brand-name medication in the U.S. since 2002. Since 2016, 10 Humira biosimilars have been approved in the U.S., which are highly similar to Humira and vary in doses, dosage forms, and concentrations.
Eisai – Biopharmaceutical Solutions
Domain: eisai.com
Registered: 1994 ( 31 years )
Introduction: HUMIRA® Pen, an auto-injector formulation for fully human anti-TNF-α monoclonal antibody HUMIRA®.
Opb – Autoimmune Disease Treatment Solutions
Domain: opb.org
Registered: 1995 ( 30 years )
Introduction: Humira, a blockbuster drug developed by AbbVie, primarily used for treating autoimmune diseases.
Rxpharmacycoupons – Humira Savings Card Solutions
Domain: rxpharmacycoupons.com
Registered: 2011 ( 14 years )
Introduction: Humira Manufacturer Coupon offers eligible patients the opportunity to pay as little as $5 per month for their Humira prescriptions through the Humira Complete Savings Card.
Biopharmadive – Humira and Biosimilars Insights
Domain: biopharmadive.com
Registered: 2013 ( 12 years )
Introduction: Humira, an injectable drug approved for various inflammatory diseases, and its upcoming biosimilars.
Hyrimoz – Biosimilar Solutions for Autoimmune Conditions
Domain: hyrimoz.com
Registered: 2015 ( 10 years )
Introduction: HYRIMOZ is the #1 biosimilar to Humira® worldwide, approved to treat various autoimmune conditions.
Fierce Healthcare – Biosimilars and Health Solutions
Domain: fiercehealthcare.com
Registered: 2004 ( 21 years )
Introduction: Blue Shield of California has entered into a deal to purchase a biosimilar version of Humira directly from the manufacturer.
Boehringer Ingelheim – Biosimilars for Autoimmune Diseases
Domain: pro.boehringer-ingelheim.com
Registered: 1995 ( 30 years )
Introduction: Cyltezo is a biosimilar to Humira (adalimumab) developed by Boehringer Ingelheim, used for the treatment of various autoimmune diseases.
SingleCare – Biologic Treatments for Inflammatory Diseases
Domain: singlecare.com
Registered: 2010 ( 15 years )
Introduction: Humira is a biologic TNF blocker medicine prescribed for various inflammatory diseases, including rheumatoid arthritis and Crohn’s disease.
Fortune – Biologic Treatments for Autoimmune Diseases
Domain: fortune.com
Registered: 1994 ( 31 years )
Introduction: Humira is a biologic medication developed by AbbVie, primarily used to treat autoimmune diseases.
Pharmaceutical Manufacturer – Biologic Therapy Solutions
Domain: pharmaceuticalmanufacturer.media
Registered: 2021 ( 4 years )
Introduction: AbbVie’s Humira (adalimumab) is a key medication in the realm of biologic therapy.
Accessdata – HUMIRA Injection Solutions
Domain: accessdata.fda.gov
Registered: 2000 ( 25 years )
Introduction: HUMIRA (adalimumab) Injection, Solution for Subcutaneous use
Tevapharm – Biosimilar Solutions for Humira®
Domain: tevapharm.com
Registered: 1996 ( 29 years )
Introduction: High-concentration interchangeable biosimilar to Humira® (adalimumab) manufactured by Alvotech for Quallent Pharmaceuticals.
Pharmaceutical Accountability – Legal Action for Drug Pricing
Domain: pharmaceuticalaccountability.org
Registered: 2019 ( 6 years )
Introduction: Humira (adalimumab) is a medication used to treat various autoimmune diseases, and the Pharmaceutical Accountability Foundation is taking legal action against AbbVie for overcharging the Dutch healthcare system for this drug.
Fierce Pharma – AbbVie Humira Biosimilars
Domain: fiercepharma.com
Registered: 2007 ( 18 years )
Introduction: AbbVie’s Humira and its biosimilars.
Ir – High-Concentration Biosimilars Solutions
Domain: ir.tevapharm.com
Registered: 1996 ( 29 years )
Introduction: High-Concentration Citrate-Free Interchangeable Biosimilar to Humira (adalimumab)
Cardinal Health – Humira® Biosimilar Solutions
Domain: cardinalhealth.com
Registered: 1996 ( 29 years )
Introduction: Humira® Biosimilar offered by Cardinal Health.
Aishealth – White-Label Biosimilars Solutions
Domain: aishealth.mmitnetwork.com
Registered: 2014 ( 11 years )
Introduction: The company primarily offers white-label biosimilars for Humira (adalimumab) through various private-label affiliates of major PBMs.
Celltrion – Biosimilars for Inflammatory Conditions
Domain: celltrion.com
Registered: 2002 ( 23 years )
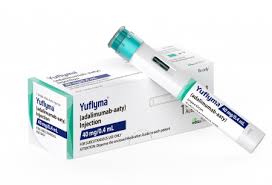
Introduction: YUFLYMA® (adalimumab-aaty) is a high-concentration (100mg/mL) and citrate-free formulation of Humira® (adalimumab) biosimilar, approved for multiple inflammatory indications.
Cvsspecialty – Humira Biosimilars for Autoimmune Conditions
Domain: cvsspecialty.com
Registered: 2014 ( 11 years )
Introduction: Humira and its FDA-approved biosimilars for various autoimmune conditions.
Category Information
The keyword “Humira manufacturer” pertains to the pharmaceutical industry, specifically focusing on the production of Humira, a leading biologic medication used to treat various autoimmune conditions such as rheumatoid arthritis, psoriasis, and Crohn’s disease. The manufacturer of Humira, AbbVie Inc., plays a crucial role in the development, production, and distribution of this drug, which has significantly impacted the treatment landscape for patients suffering from chronic inflammatory diseases.
The significance of the manufacturer category extends beyond just the production of the drug; it encompasses regulatory compliance, quality control, and ongoing research and development efforts. Manufacturers must adhere to strict regulations set by health authorities to ensure the safety and efficacy of their products. Furthermore, the competitive landscape is evolving, especially with the introduction of biosimilars, which are designed to offer more affordable treatment options, thereby influencing market dynamics and patient access to essential medications.
Application Information
Humira, manufactured by AbbVie, is primarily used in the healthcare industry, particularly for treating autoimmune diseases. One of its key application areas is in the management of conditions such as rheumatoid arthritis and Crohn’s disease, where it helps reduce inflammation and alleviate symptoms, improving patients’ quality of life. Another significant application area is dermatology, where Humira is employed to treat psoriasis and hidradenitis suppurativa, providing relief from chronic skin conditions.
Additionally, it is used in gastroenterology for ulcerative colitis, showcasing its versatility in treating various inflammatory conditions. These applications highlight Humira’s critical role in modern therapeutic regimens, significantly impacting patient care across multiple specialties.
Production Process Information
The production process for Humira, a medication used to treat various autoimmune conditions, involves several key stages. First, the research and development phase is crucial, where scientists identify the specific proteins that target disease pathways. This phase includes extensive testing to ensure the drug’s effectiveness and safety. Once the formula is finalized, the manufacturing stage begins. This involves cultivating living cells that produce the desired antibodies, which are harvested and purified.
Quality control is vital during this stage to ensure that the final product meets strict safety standards. Finally, the packaging and distribution phase ensures that Humira reaches pharmacies and healthcare providers. Throughout this process, regulatory compliance is essential to meet government and industry standards, ensuring that patients receive safe and effective medication.
Related Video
Frequently Asked Questions (FAQs)
What should I look for in a Humira manufacturer?
When choosing a Humira manufacturer, prioritize factors such as regulatory compliance, quality certifications, production capacity, and experience in biologics. It’s also important to assess their supply chain reliability and customer service, ensuring they can meet your specific needs and timelines.
How can I verify a manufacturer’s credentials?
You can verify a manufacturer’s credentials by checking their certifications, such as FDA approval or EMA compliance. Additionally, request references from other clients and look for third-party audits or inspections that demonstrate their adherence to industry standards.
What questions should I ask during a factory visit?
During a factory visit, ask about their production processes, quality control measures, and handling of biologics. Inquire about their staff training, equipment maintenance, and how they manage potential contamination risks. Observing their operations firsthand can provide valuable insights.
How do I assess the cost of manufacturing Humira?
To assess manufacturing costs, request detailed quotes from multiple suppliers, including breakdowns of production, materials, and shipping expenses. Consider the total cost of ownership, which includes potential hidden costs like regulatory fees or quality assurance expenses.
What are the common challenges in sourcing Humira manufacturers?
Common challenges include ensuring compliance with stringent regulations, maintaining consistent quality, and managing lead times. Additionally, navigating complex supply chains and potential language barriers can complicate communication. It’s essential to build strong relationships with suppliers to overcome these hurdles.