Top 30 Stelara Manufacturers for Autoimmune Treatments
Are you struggling to find a reliable manufacturer for Stelara? You’re not alone! With so many options out there, it can feel overwhelming to choose the right factory that meets your quality and cost expectations. Finding the best supplier can make all the difference in ensuring consistent product quality and timely delivery, saving you time and headaches down the road. Imagine partnering with a top-notch factory that boosts your confidence and enhances your business reputation!
In this article, we’ve curated a list of the top 30 Stelara manufacturers, highlighting their strengths and specialties. Ready to simplify your search and elevate your supply chain? Dive in and discover the best of the best!
Top 30 Stelara Manufacturer Manufacturers
Stelarainfo – Psoriasis and Crohn’s Disease Treatment
Domain: stelarainfo.com
Registered: 2008 ( 17 years )
Introduction: STELARA® (ustekinumab) is a prescription medicine used to treat moderate to severe psoriasis, active psoriatic arthritis, moderately to severely active Crohn’s disease, and moderately to severely active ulcerative colitis in adults and children 6 years and older.
Stelarahcp – Immunosuppressant Treatment Solutions
Domain: stelarahcp.com
Registered: 2014 ( 11 years )
Introduction: STELARA® (ustekinumab) is an immunosuppressant medication used for the treatment of plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
Jnjmedicalconnect – Autoimmune Treatment Solutions
Domain: jnjmedicalconnect.com
Registered: 2024 ( 1 years )
Introduction: STELARA® (ustekinumab) is a medication used for the treatment of certain autoimmune conditions.
Healio – Stelara Biosimilars Solutions
Domain: healio.com
Registered: 2010 ( 15 years )
Introduction: First four Stelara biosimilars launched in the US.
Jnj – Ulcerative Colitis Treatment Solutions
Domain: jnj.com
Registered: 1993 ( 32 years )
Introduction: Stelara (ustekinumab) is approved for the treatment of adults with moderately to severely active ulcerative colitis.
Drugs.com – Biologic Treatments for Autoimmune Conditions
Domain: drugs.com
Registered: 1998 ( 27 years )
Introduction: Stelara is a biologic medication used for the treatment of certain autoimmune conditions, including psoriasis, psoriatic arthritis, and Crohn’s disease.
Fda – STELARA® Treatment Solutions
Domain: fda.report
Registered: 2018 ( 7 years )
Introduction: STELARA® (ustekinumab) is a prescription medication indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, moderately to severely active Crohn’s disease, and moderately to severely active ulcerative colitis.
Biocon – Biosimilars for Autoimmune Diseases
Domain: biocon.com
Registered: 1996 ( 29 years )
Introduction: YESINTEK™ (ustekinumab-kfce) is a biosimilar to Stelara® (ustekinumab) approved for the treatment of Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis.
Steqeyma – Biosimilars for Chronic Inflammatory Diseases
Domain: steqeyma.com
Registered: 2022 ( 3 years )
Introduction: STEQEYMA is a biosimilar to Stelara® (ustekinumab) approved for the treatment of plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
Pharmaceutical Technology – Biosimilar Development Solutions
Domain: pharmaceutical-technology.com
Registered: 2000 ( 25 years )
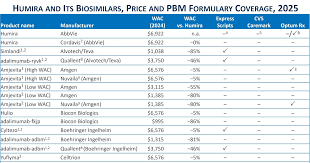
Introduction: Teva and Alvotech have collaborated to develop a biosimilar product named Selarsdi, which is a biosimilar to AbbVie’s Humira (adalimumab).
Tevapharm – Biosimilars for Psoriasis and Arthritis
Domain: tevapharm.com
Registered: 1996 ( 29 years )
Introduction: SELARSDI (ustekinumab-aekn) injection is a biosimilar to Stelara, approved for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis in adults and pediatric patients 6 years and older.
Aishealth – Biosimilar Solutions for Stelara
Domain: aishealth.mmitnetwork.com
Registered: 2014 ( 11 years )
Introduction: Wezlana (ustekinumab-auub) is the first biosimilar of Stelara (ustekinumab) and is distributed solely by Optum’s Nuvaila.
M – Ustekinumab Injections for Chronic Conditions
Domain: m.indiamart.com
Registered: 1996 ( 29 years )
Introduction: The company primarily offers Ustekinumab injections, including various formulations such as STELARA in different strengths (90 mg, 130 mg, and 45 mg) for the treatment of conditions like Plaque Psoriasis, Psoriatic Arthritis, Ulcerative Colitis, and Crohn’s Disease.
Accessdata – STELARA® Injection for Autoimmune Conditions
Domain: accessdata.fda.gov
Registered: 2000 ( 25 years )
Introduction: STELARA ® (ustekinumab) injection, for subcutaneous or intravenous use, indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, moderately to severely active Crohn’s disease, and moderately to severely active ulcerative colitis.
Cvsspecialty – Biosimilars for Stelara
Domain: cvsspecialty.com
Registered: 2014 ( 11 years )
Introduction: Biosimilars for Stelara, including Pyzchiva, Selarsdi, Steqeyma, and Yesintek, as well as the original Stelara (ustekinumab).
Cencora – Biosimilar Development and Commercialization Solutions
Domain: cencora.com
Registered: 2017 ( 8 years )
Introduction: Cencora offers a comprehensive suite of services to support the development, commercialization, and distribution of biosimilars, specifically focusing on the Stelara biosimilar landscape.
Dir – Ustekinumab Injection for Autoimmune Conditions
Domain: dir.indiamart.com
Registered: 1996 ( 29 years )
Introduction: Ustekinumab Injection, marketed under the brand name Stelara, is used to treat autoimmune conditions such as psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
Ir – Biosimilar Therapeutics for Autoimmune Diseases
Domain: ir.tevapharm.com
Registered: 1996 ( 29 years )
Introduction: SELARSDI (ustekinumab-aekn) Injection is a biosimilar to Janssen’s Stelara, indicated for the treatment of certain autoimmune diseases.
Uhcprovider – Psoriasis and Crohn’s Disease Treatments
Domain: uhcprovider.com
Registered: 2002 ( 23 years )
Introduction: Stelara (ustekinumab), Steqeyma (ustekinumab-stba), Yesintek (ustekinumab-kfce) are medications indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
Fierce Pharma – Biosimilars for Autoimmune Treatments
Domain: fiercepharma.com
Registered: 2007 ( 18 years )
Introduction: Amgen’s biosimilar version of Johnson & Johnson’s Stelara, which is used for treating various autoimmune conditions.
Ema – Autoimmune Disease Treatment Solutions
Domain: ema.europa.eu
Registration year: Not available
Introduction: Stelara is a medication used for the treatment of various autoimmune diseases, including psoriasis, psoriatic arthritis, and Crohn’s disease.
Wezlana – Biosimilars for Managed Healthcare
Domain: managedhealthcareexecutive.com
Registered: 2000 ( 25 years )
Introduction: Wezlana is the first biosimilar to Stelara (ustekinumab) and is available exclusively through Optum’s private label.
FDA – STELARA® for Plaque Psoriasis Treatment
Domain: fda.gov
Registered: 2000 ( 25 years )
Introduction: STELARA ® (ustekinumab) is a human monoclonal antibody indicated for the treatment of adult patients (18 years or older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
PrescriptionGiant – Prescription Savings Program
Domain: prescriptiongiant.com
Registered: 2005 ( 20 years )
Introduction: PrescriptionGiant offers a free prescription savings program that can save users up to 75% on prescriptions, including specific discounts for Stelara through manufacturer coupons.
Rxpharmacycoupons – Stelara Manufacturer Discount Program
Domain: rxpharmacycoupons.com
Registered: 2011 ( 14 years )
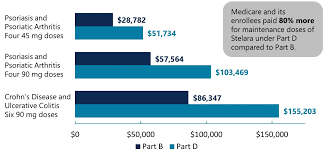
Introduction: Stelara Manufacturer Coupon offers eligible patients the opportunity to pay only $5 per dose of Stelara, with a maximum program benefit of $20,000 per calendar year.
Selarsdipp – Psoriasis and Psoriatic Arthritis Treatment
Domain: selarsdipp.tevapharm.com
Registered: 1996 ( 29 years )
Introduction: SELARSDI™ (ustekinumab-aekn) injection is a human interleukin-12 and -23 antagonist indicated for the treatment of adults and pediatric patients 6 years of age and older with moderate to severe plaque psoriasis and active psoriatic arthritis.
Secure – Affordable Biologics for Chronic Conditions
Domain: secure.medicalletter.org
Registered: 1998 ( 27 years )
Introduction: Information not available.
OIG – Autoimmune Treatment Solutions
Domain: oig.hhs.gov
Registered: 1997 ( 28 years )
Introduction: Stelara is a medication used for the treatment of certain autoimmune conditions, including psoriasis and Crohn’s disease.
Center for Biosimilars – Affordable Biosimilars for Patients
Domain: centerforbiosimilars.com
Registered: 2016 ( 9 years )
Introduction: Wezlana is the first biosimilar referencing Stelara (ustekinumab) launched in the US, providing lower-cost biologics for patients with rheumatic and gastrointestinal conditions.
Pharmabiz – Biosimilars and Injectables Solutions
Domain: pharmabiz.com
Registered: 2000 ( 25 years )
Introduction: Starjemza (ustekinumab-hmny) Injection, a biosimilar referencing Stelara (ustekinumab) Injection.
Category Information
The keyword ‘Stelara manufacturer’ pertains to the pharmaceutical industry, specifically focusing on the production of Stelara, a biologic medication used to treat autoimmune conditions such as psoriasis, Crohn’s disease, and ulcerative colitis. Stelara is a monoclonal antibody that works by inhibiting specific proteins in the immune system, thereby reducing inflammation and the symptoms associated with these chronic diseases. The manufacturer plays a crucial role in ensuring the safety, efficacy, and availability of this medication to patients in need.
The significance of identifying the manufacturer of Stelara lies in understanding the broader implications of drug production, including regulatory compliance, quality control, and the ethical responsibilities involved in delivering healthcare solutions. Moreover, knowing the manufacturer can also provide insights into ongoing research and development efforts, as well as potential support resources available for patients undergoing treatment. This information is vital for healthcare professionals, patients, and stakeholders within the healthcare system.
Application Information
Stelara, manufactured by Janssen Pharmaceuticals, is primarily used in the treatment of autoimmune diseases, making it relevant in several key application areas. One of the most significant applications is in dermatology, where Stelara is utilized for managing moderate to severe plaque psoriasis. This helps improve patients’ skin conditions and overall quality of life. Another critical area is gastroenterology, as Stelara is effective for treating Crohn’s disease and ulcerative colitis.
These inflammatory bowel diseases can severely impact daily living, and Stelara offers a therapeutic option for long-term management. Additionally, Stelara is used in rheumatology for treating psoriatic arthritis, providing relief from joint pain and inflammation. Overall, Stelara’s applications span across various medical specialties, significantly enhancing patient outcomes in chronic autoimmune conditions.
Production Process Information
Stelara, a medication used to treat autoimmune conditions, is produced through a detailed manufacturing process. The key steps involve several crucial stages, ensuring the final product is safe and effective for patients. First, the initial stage involves the development of the drug’s active ingredient, known as the biologic. This requires advanced biotechnology methods, including cell culture techniques to grow the necessary cells that produce the drug. Once the active ingredient is created, it undergoes rigorous testing for quality and effectiveness.
Next, the formulation stage combines the active ingredient with other components to create the final injectable product. After formulation, the medication is filled into vials or syringes in a sterile environment. Finally, the product is packaged, labeled, and distributed to healthcare providers, ensuring patients receive their medication safely. Throughout the entire process, strict regulatory guidelines are followed to maintain quality and safety standards.
Frequently Asked Questions (FAQs)
What should I consider when choosing a Stelara manufacturer?
When selecting a Stelara manufacturer, consider factors like their experience in biologics, regulatory compliance, quality control measures, and production capacity. It’s also important to evaluate their track record for timely delivery and customer service, as well as their ability to meet your specific needs.
How can I verify a Stelara manufacturer’s credentials?
You can verify a manufacturer’s credentials by checking their certifications, such as GMP (Good Manufacturing Practices) and any relevant FDA approvals. Additionally, request references from previous clients and review their history of compliance with industry regulations to ensure they meet high standards.
What are the benefits of working with an established Stelara manufacturer?
Working with an established manufacturer often means better reliability, access to advanced technology, and a wealth of experience in handling complex production processes. They are more likely to have robust quality assurance systems in place, which can lead to higher product consistency and safety.
How do I assess the quality of a Stelara manufacturer’s products?
To assess product quality, request samples and conduct thorough testing. Review their quality control protocols, including batch testing and stability studies. You can also look for third-party certifications and audit reports that demonstrate their commitment to maintaining high-quality standards.
What is the typical lead time for Stelara manufacturing?
Lead times can vary based on the manufacturer and your specific requirements, but typically, you should expect anywhere from a few months to over a year for production. It’s best to discuss timelines upfront and factor in additional time for quality checks and regulatory approvals.